Led by Dr. Zhang, NanoHELP’s research team is dedicated to being on the forefront of sustainable, affordable energy research through core skills in thermal-fluid sciences and combustions, micro-/nano- technology, advanced manufacturings, 3-D printings, and state-of-the-art spectroscopies and diagnostics. Through a robust interdisciplinary program, we explore the intricacies of micro/nano-scale chemical reactions, delve into the complexities of heat/mass transport, and unravel the mysteries of fluid mechanics. See examples of our work below for insight into the research conducted within the NanoHELP lab.
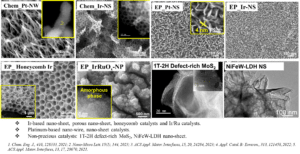
Tuning nanostructure/composition/chemical state/crystal phase of catalyst on various PTL substrates.

CO2 capture and reduction to high-value formic acid with electrolyzers.


N2 reduction to NH3 under atmospheric environment with electrolyzerss.


Thin and tunable LGDL Application in Alkaline Anion Exchange Membrane (AEM) Electrolyzer cells.

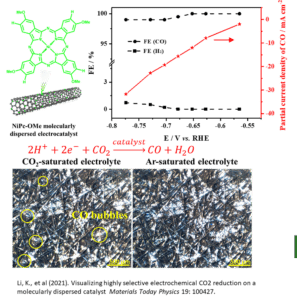
CO2 capture and reduction to high-value CO with electrolyzers.

Enhancing Catalyst Conductivity with Conductive Mesoporous Bi-metallic Catalysts (CMBMCs).


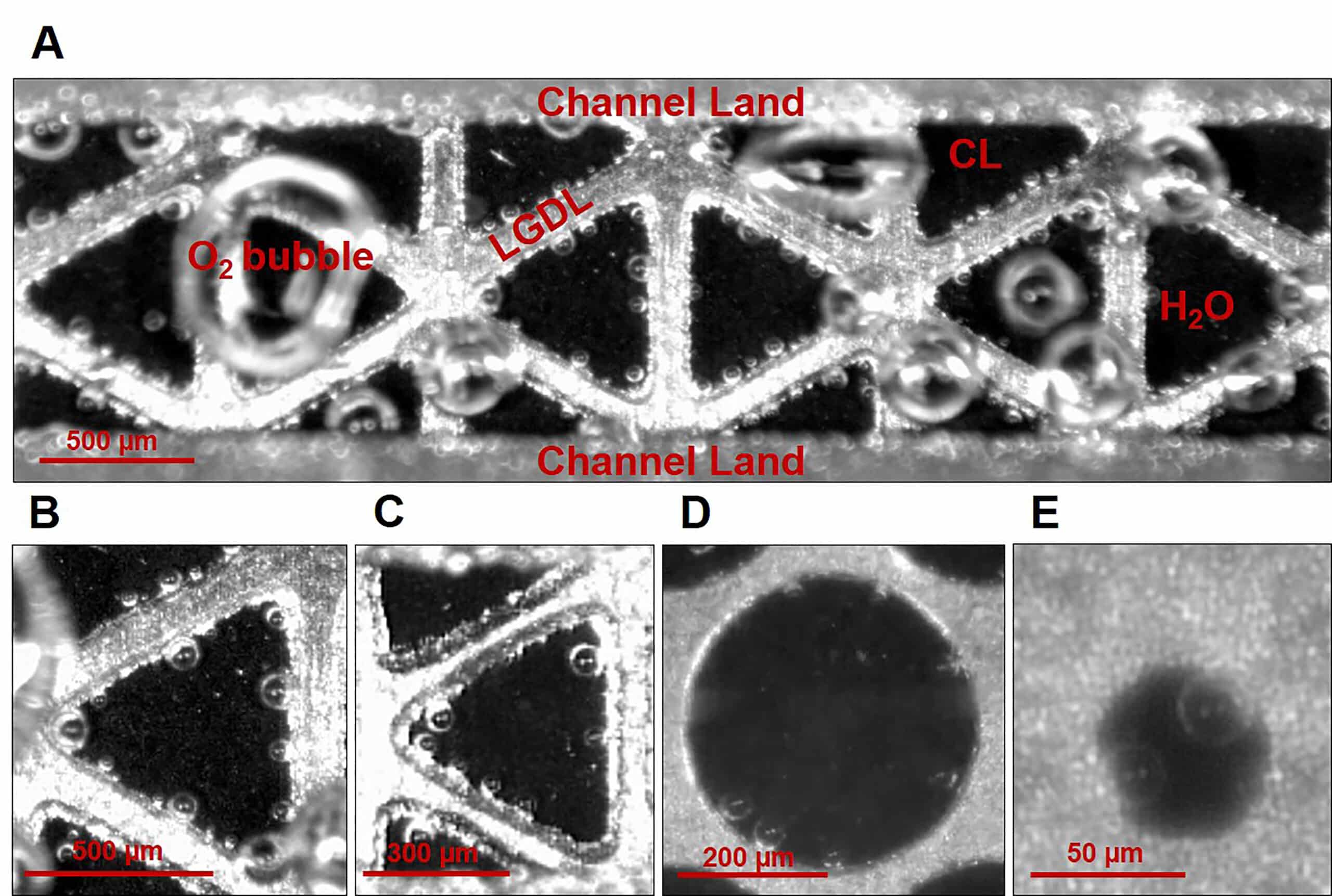
- (A) Electrochemical reactions in the PEMEC microchannel.
- (B) 600 µm triangular opening.
- (C) 400 µm triangular opening.
- (D) 500 µm circular opening.
- (E) 50 µm circular opening.
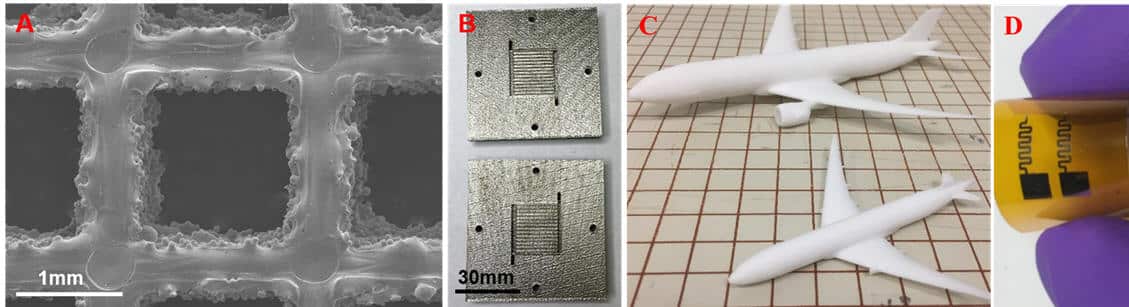
- (A) Titanium liquid/gas diffusion layer by electron-beam melting
- (B) Stainless steel bipolar plate by selective laser melting
- (C) Nonmetallic airplane by binder-jetting prototype
- (D) Circuits/sensors by direct printing
Thin and well tunable electrodes with ultrahigh catalyst mass activity in water splitting.
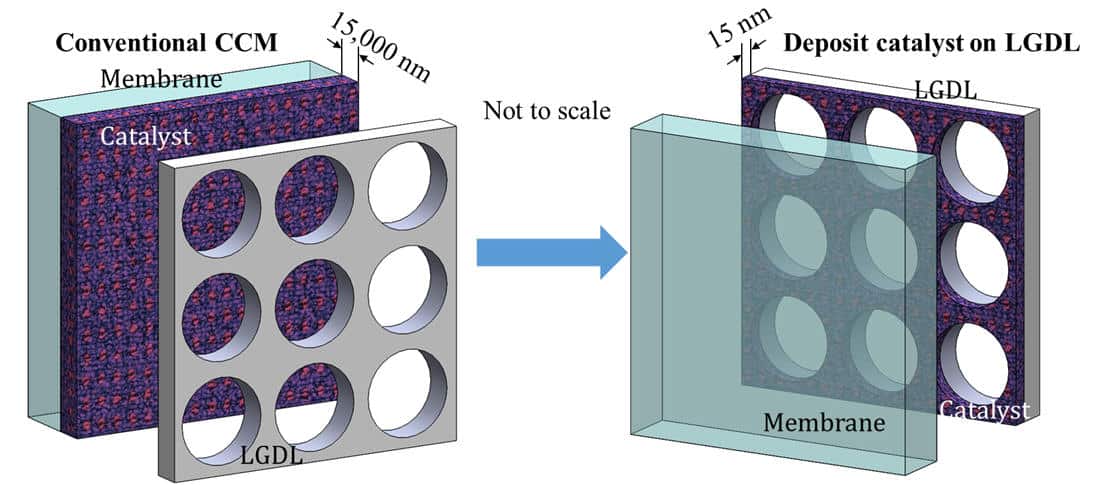

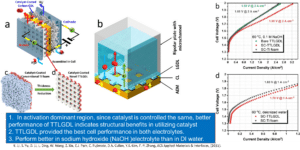
Thin/well-tunable liquid/gas diffusion layers exhibiting superior multifunctional performance for hydrogen production in low-temperature electrolytic water splitting.


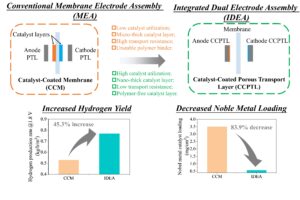
Integrated Dual Electrode Assembly (IDEA) demonstrates a significantly higher performance, lower noble metal loading, and more facile manufacturing.
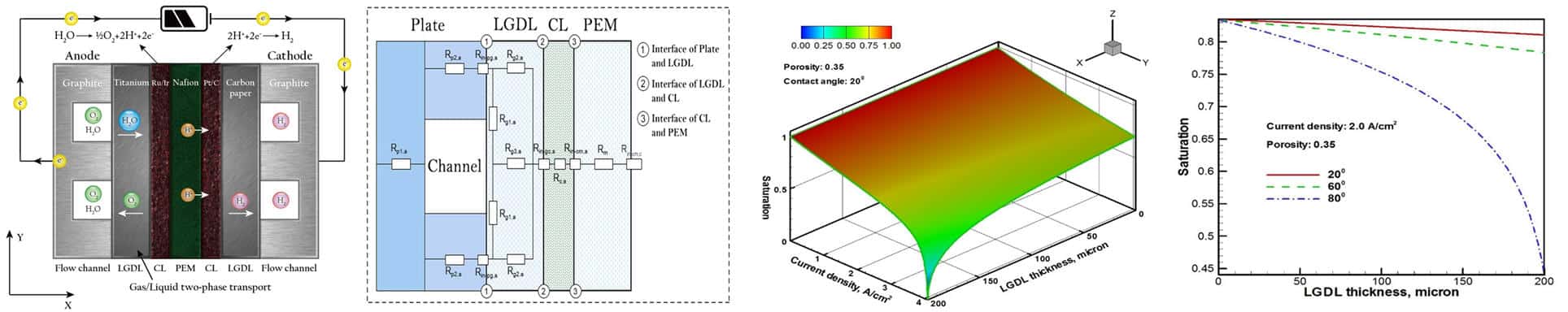
Modeling and simulation of multiphase transport, interfacial effects and electrochemical performance.

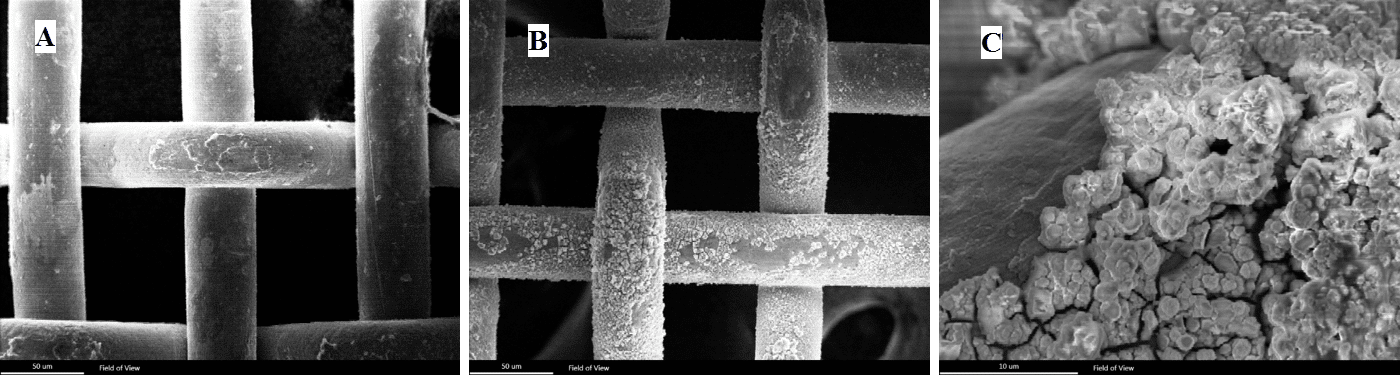
SEM images of stainless steel corrosions:
- (A) fresh sample
- (B) used sample
- (C) Close-up of (B)
SEM images of corrosion deposition on carbon fiber
- (A) fresh sample
- (B) used sample

- (A) fresh sample
- (B) used sample

- (A) in flow channel and on GDL
- (B) in GDL
- (C) on catalyst layer and reaction site

- (A) in hydrophobic
- (B) in hydrophilic

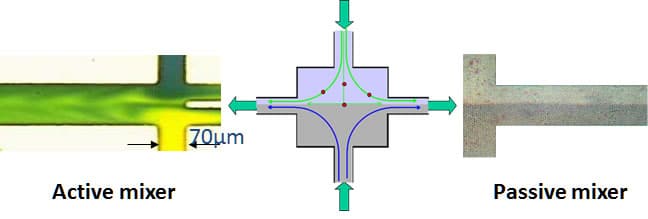
Mixing within less than 0.5 mm in active mixer (left), while no mixing observed in passive mixer (right).

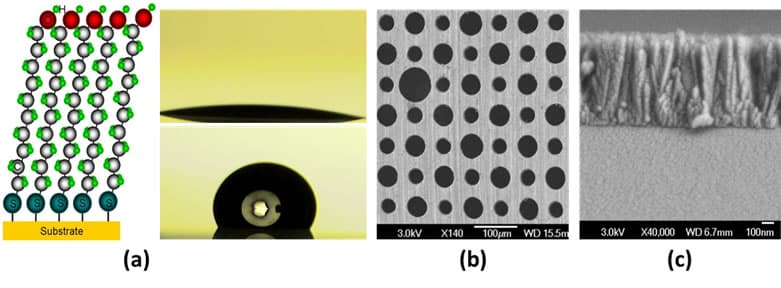
- (A) Molecular self-assembled monolayer (SAM)
- (B)Top-down/etching
- (C) Bottom-up/deposition
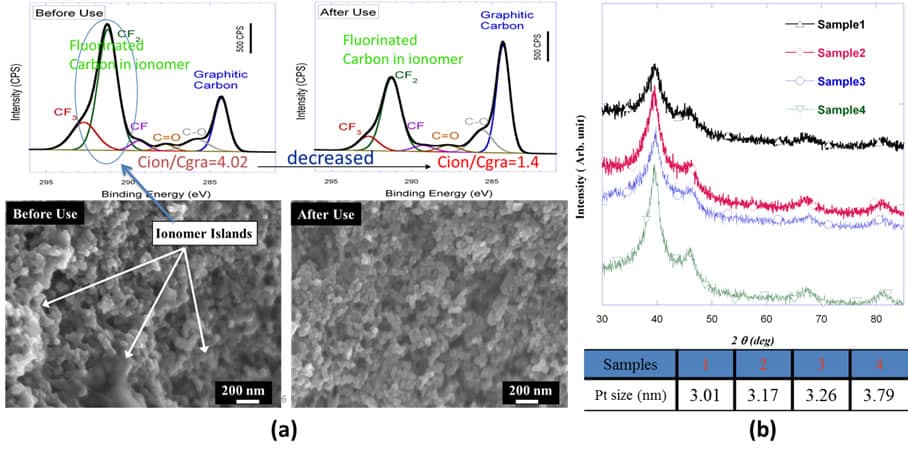
- (A) by x-ray photoelectron spectroscopy and electron microscopy
- (B) x-ray diffraction
Pulse detonation engines at operating frequency of up to 32 Hz with air/H2. The image shows the wave out of engine exit.


Characterizations of PEM water electrolyzers with different materials:
- (A) Performance
- (B) Electrochemical impedance spectroscopy
- (C) EIS equivalent electrical circuit model



